Reference Toxicant Testing
Reference toxicant tests are an important part of ETS's toxicity testing program. They serve to:
- determine the sensitivity of the test organisms over time
- assess comparability within and between laboratory test results
- identify potential sources of variability, such as test organism health, differences among batches of organisms, changes in laboratory water or food quality, and performance by laboratory analysts
Control charts are used to monitor the ongoing sensitivity, precision and accuracy of toxicity tests performed in the laboratory. Charts are prepared for each combination of reference toxicant, test species, test condition, and endpoint.
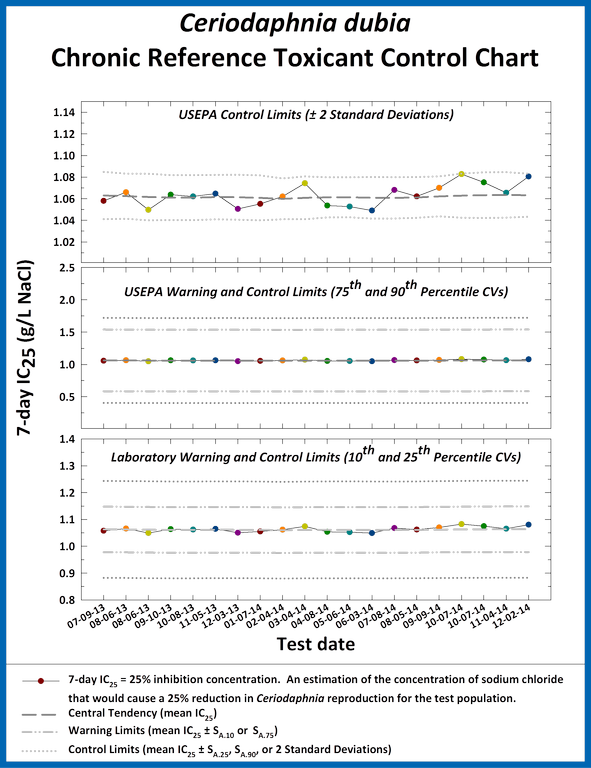
Example Ceriodaphnia dubia chronic reference toxicant control chart.
Precision
Toxicity test precision is based on control coefficient of variation (CV) and the Percent Minimum Significant Difference (PMSD). These measures of test precision quantify within-test variability, or the sensitivity of each test to toxic effects on the biological endpoint. In addition, CV indicates variability among replicates under non-toxic conditions and is an indicator of uniformity of the test organisms
Percent minimum significant difference is used to assess the overall precision in the toxicity tests performed in the laboratory. Reference toxicant tests are used to characterize method variability because, in contrast to effluent samples, fixed concentrations of known toxicants are used. Only with this standardization is it possible to conclude that variability of the effect concentration estimates is derived from the sources discussed above, rather than from changes in the toxicant. These reference toxicant tests are conducted using the same test conditions (type of dilution water, temperature, test protocol, and species) that are used for toxicity tests conducted by ETS.
Control charts are used to monitor the ongoing precision through PMSDs at ETS. Upper PMSD bounds established by the USEPA are impractical for use in our laboratory due to the large limits. As a result, ETS has established acceptance limits based on control charts using two standard deviations (95% confidence intervals) from the mean of 20 data points.
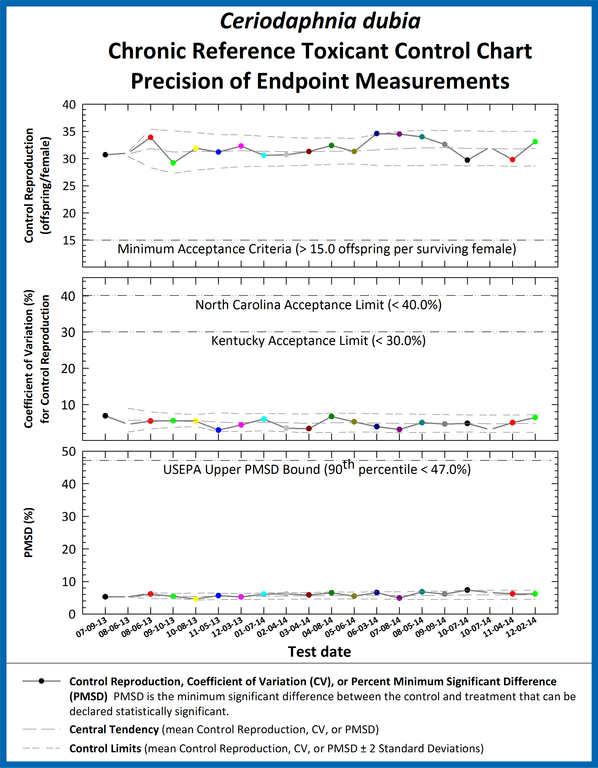
Example Ceriodaphnia dubia chronic reference toxicant control chart, providing estimates of test precision.
